Painting on galvanized steel = Duplex system
Many analyses have shown that a duplex system, i.e. hot-dip galvanizing plus painting , is a very cost effective way to protect steel, particularly with regard to its service life up to the first re-painting. Although hot-dip galvanizing alone usually gives fully satisfactory protection against corrosion, additional protection is sometimes desirable in aggressive environments. It can, for example, be advantageous if future maintenance is likely to be difficult to carry out, or if the zinc coating is thin, as on continuous galvanized thin sheet steel.
Aesthetic reasons — giving the matt grey zinc surface a more pleasing appearance — can also play a role. Similarly, paint can be used to give prominence to or warn of the existence of a structure, or even the opposite — to camouflage the structure. Insulation against galvanic corrosion if the galvanized steel is to be connected to another metal, such as copper, can be another reason for painting.

Service life of the duplex system
A duplex system generally has a much longer service life than each individual coating. Dutch experiments show that the service life of the system can be calculated according to the following formula:
LT = K(LZn+Lp)
where
LT = the service life of the duplex system in years
LZn = the estimated service life of the zinc coating in years (in the actual environment)
Lp = the estimated service life of the paint coating in years (in the actual environment), if it is applied directly to steel
K= environment-dependent factor between1,5 and 2,3:
- 1,5 when the system is exposed in environmental class C5 (see fig 2-2) or in sea-water.
- 1,6 – 2,0 when the system is exposed in environmental class C3 – C4 or when the wet period for the system is lower than 60 %.
- 2,1- 2,3 when exposed in environmental class C2.
Of course, the galvanized surface must have been correctly pretreated to ensure lasting paint adhesion. To obtain this, the zinc surface must be cleaned thoroughly and the correct type of paint must be used. The painting of zinc-coated surfaces requires somewhat more care than some other materials. Small quantities of impurities on the zinc surface, or the incorrect paint type, can lead to premature blistering and/or flaking.
It was often recommended in the past that the zinc surface should be exposed outdoors for 1-2 years before painting. The recommendation was correct before 1950. At that time the atmosphere, at least in Scandinavian countries, was relatively clean, which meant that the corrosion products that formed on a zinc surface consisted almost entirely of basic zinc carbonates.
Therefore, after a period of exposure, paint was not applied to a reactive zinc surface but to an inert layer of carbonates. The result was generally good, even with paints which would today be regarded as unsuitable. Today this method is suspect since the atmosphere contains sulphur oxides pollution and the corrosion products of zinc often contain water-soluble zinc salts. Paint applied to such a layer, which is to some extent water-soluble, will probably blister or flake, regardless of the paint system.
Newly-galvanized, shiny surfaces
A shiny zinc surface is often considered to be clean enough to paint. In many cases this is not true, and this leads to poor results.
A freshly galvanized surface is a good substrate for painting only if:
• The article has not been water quenched. Quench water is seldom clean. Different salts that may be deposited on the zinc surface can later decrease or totally destroy the adherence of the paint coating.
• The article has not been stored inside the galvanizing plant. The air in the plant contains flux dusts (particles of zinc and ammonium chloride). These particles adhere to the surface and create a water soluble film which reduces the adherence.
• The article has not been stored or transported outdoors in a damp atmosphere. The risk of condensation, which leads to wet storage strain, is significant. It is not always visible to the naked eye, but will cause problems after painting.
• The article has not been stored more than about six hours between galvanizing and painting. The precise critical time is, of course, dependent of the cleanliness and dryness of the surrounding air.
A “fresh” zinc surface is seldom as clean as one can be led to believe by the lustre. Thin films of oil and grease from gloves, shoes and hoisting slings can increase the impression of a shiny and clean surface. Contamination exists in thin films which are transparent and very difficult to spot with the naked eye.
Hot-dip galvanized thin sheet is often chromated or oiled for protection against wet-storage stain. The chromate layer is somewhat water soluble, and painting over a film of oil seldom meets with success. Layers such as these must therefore be removed before painting. Chromating is, for environmental reasons, nowadays replaced by other methods of passivation.
Exposed, matt surfaces
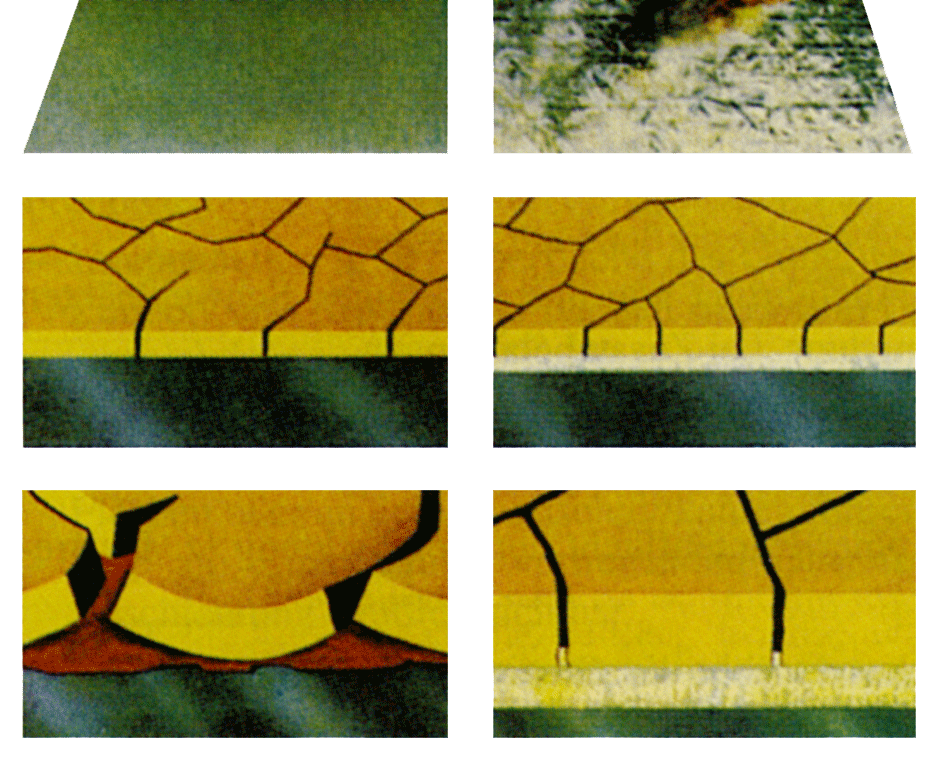
Exposed matt zinc surfaces are always covered with corrosion products. Their composition is difficult to determine. As a rule-of-thumb, it should be assumed that they contain water-soluble or hygroscopic salts which make them unsuitable substrates for painting. It follows that some form of cleaning is always necessary before matt surfaces are painted. Paint coatings are always more or less permeable to water. This means that if the water-soluble products are not removed from the zinc surface, blisters full of solution will form beneath the paint coating. Access to protective film forming substances is poor in the salt solution and results in corrosion of the zinc layer. Corrosion spreads between the paint and zinc and the corrosion products dislodge the paint coating.
Cleaning and surface preparation
All experience shows that sweep-blasting gives the best foundation for good adhesion between paint and zinc. Its moderate mechanical effect removes all corrosion products and other impurities from the surface, even the water soluble products. Agents that protect against wet-storage stain are removed effectively, as are different types of oil. Many zinc surfaces are very flat and shiny. For these, blasting is beneficial since it creates a key on the surface which enables better mechanical anchorage of the paint coating.
Recommended data for sweep-blasting of galvanized surfaces is given in the table below. It is important that the instructions given in are followed. Inadequate cleaning may impair paint adhesion while excessive mechanical working can destroy the zinc layer or cause built-in stresses, which could subsequently cause the paint coating to flake.
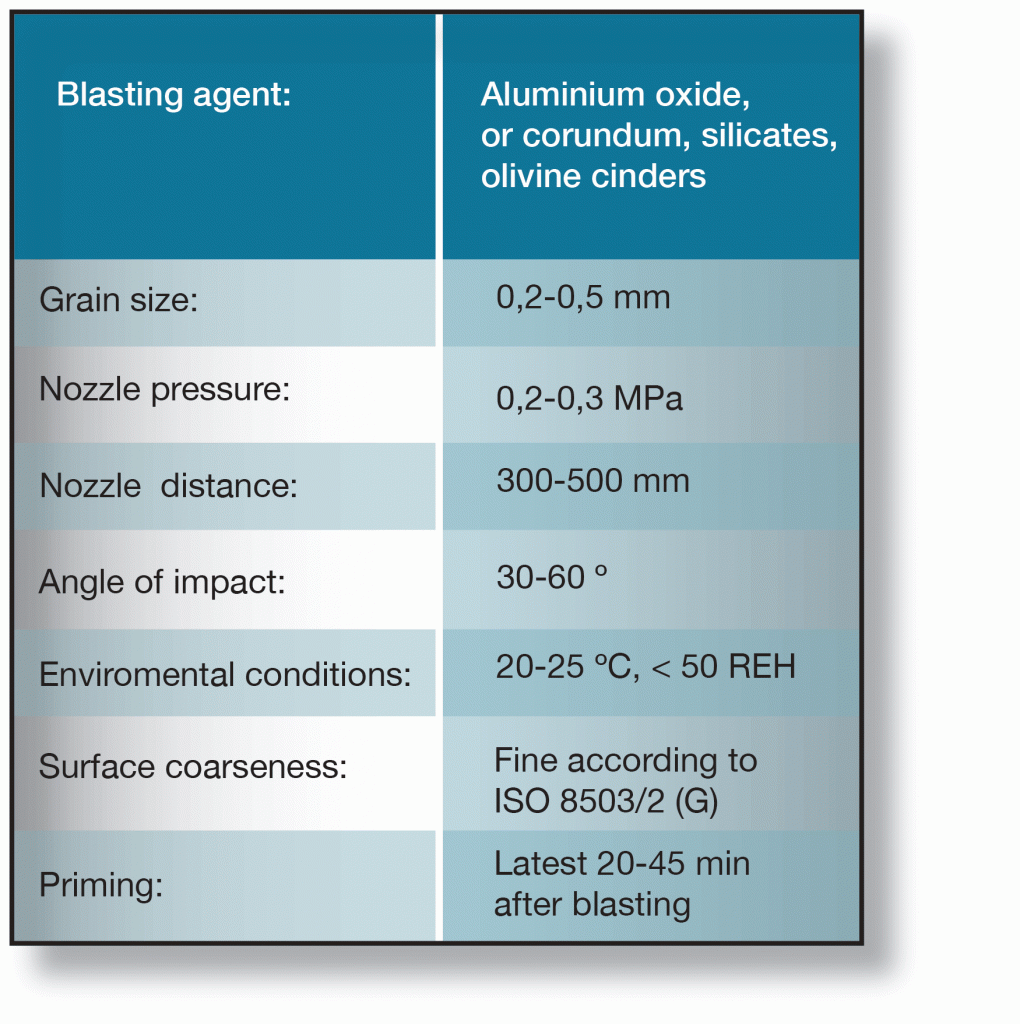
When sweep-blasting is performed correctly about 10 µm of the zinc layer is removed. If sweep-blasting cannot be used industrial lacquering according to the following procedure is recommended:
1. Alkaline degreasing
2. Thorough water washing
3. Phosphating (iron or zinc phosphate)
4. Thorough water washing
5. Drying
6. Lacquering
Chromated goods require alkaline degreasing and sometimes brushing, or treatment with nylon wool impregnated with a grinding agent of aluminium oxide. The phosphate layer should be as thin as possible, but should give continuous full cover of 2 – 4 g/m2. A thickness under 1 µm is usually considered to be optimal.
In the case of manual painting, degreasing with an emulsifying degreasing agent is recommended, preferably combined with brushing or lapping with nylon wool and thorough rinsing with water – high pressure if possible.
Some commercial degreasing agents contain oxide-dissolving additives. This can be an advantage when the composition of the corrosion products is not known.
Choice of paint
When choosing paint, it should be remembered that a paint can contain up to 10—15 different components. Each manufacturer has his own formula for a given type. Suppliers of raw materials also have different formulae for binding agents, which means that the number of variations can be very great. Paints of the same type, but from different manufacturers, can therefore have different properties. The guidance below should therefore be regarded as general guidelines only. If in doubt, discuss your selection of paint with the manufacturer’s representatives. Where hot-dip galvanized surfaces are to be painted, it is recommended that the painting systems in EN ISO 12944-5: 2018 are used. Painting of hot-dip galvanized goods is stated in Table D.1 This table describes suitablepainting system for corrosivity class C2 – C5, with different durability times. The painting systems have the designation “G” (Galvanized). “
Powder Coating
Different types of powder coatings are becoming more common on hot-dip galvanized steel. If the requirements on surface finish is very high, the whole surface can be grinded to remove all tags and drops that can interfere with the appearance. In addition, grinding gives very clean surface with the best conditions for optimum adhesion. The galvanized steel pass through a number of pretreatment baths before powder coating. Degreasing, rinsing, etching, rinsing, phosphating and sealing are examples of process steps that can be included. After that the steel has to try. The powder is applied electrostatically, and after that the steel is transported into the curing oven.
Pinholes
Pinholes are small holes in the coating layer, which can vary in size from small to larger pores. Pinholes is primarily an aesthetic problem. The corrosion protection is still good because of the good protective effect from zinc, but the surfaces may look less appealing. The main reason for pinholes to form is presence of moisture and white rust on the galvanized surface, which give rise to gas formation during the curing process.
To avoid / reduce the risk of pinholes, the following should be considered: •
The zinc surface should be free from contaminants and roughness.
• Avoid exposing the goods in a damp environment – avoid white rust formation.
• Select the right steel for galvanizing – any material with low silicon content, below 0:03% – which gives a smoother surface and also is more durable when blasting.
• Ensure that the phosphate layer, which is applied before powder coating, has had time to dry properly.
• Preheat the goods.
• Use of special powder quality which counteracts the formation of pinholes.
• Use powder with low viscosity, which easily floats out.
• Thicker layer of powder paint can in some cases cause the pores not to penetrate the layer, and therefore not give visible defects.
• Raise the temperature slowly during curing of the powder coat.
Advises for duplex treatment
The galvanizer need to know in advance if the steel will be duplex treated, since this means extra demands on the surfaces. Primarily use steel with a silicon content that gives a zinc coating with a layer of pure zinc at the surface. This type of coating can withstand the blasting better. If silicon killed steel has already been used – inform the painter about that. If painting is carried out by another company than the galvanizer, check that the right skills and equipment are available.

