Reinforced concrete is an important construction material. Highway bridges, car parks, houses, office buildings, gulleys are examples of reinforced concrete structures. In many cases, its steel reinforcement does not need any external corrosion protection to avoid corrosion. The highly alkaline environment of concrete forms a thin oxide layer on the steel surface which protects or “passivates” the steel against further corrosion.
But, in some instances that passivation does not work well enough, or even not at all. This may occur when:
- The concrete has cracks, clevages, sand pockets or too little cover.
- The alkaline environment has been neutralized (carbonation).
- Chlorides have penetrated the concrete (marine environment, winter prepared roads).
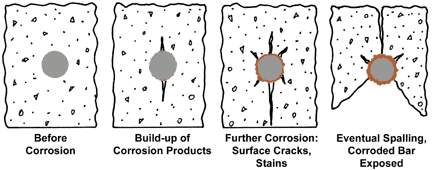
These situations often occur in the outermost parts of a construction. Defects in reinforced concrete have increased because of increased salting and atmospheric impurities. Defects in different concrete constructions are more common today than previously recognised, fig 1 . Once corrosion of the reinforcement has started it is very difficult and expensive to repair. The need to protect reinforcement from corrosion is becoming increasingly important in several concrete applications.

Barrier and cathodic protection
Galvanized reinforcing steel is bare reinforcing steel coated with a protective layer of zinc metal. The zinc coating serves as a barrier to corrosive elements that the rebar is exposed to when embedded in concrete. In addition to the barrier protection that the zinc provides, it also provides a level of cathodic protection where the zinc will preferentially corrode when in contact with bare steel (e.g. a void on the surface of the galvanized rebar will be protected by the surrounding zinc.) Galvanized coatings also possess other favorable corrosion characteristics when embedded in concrete, making it an effective coating material for corrosion resistance.

The possibility to protect reinforcing steel with hot-dip galvanizing is well documented by case histories in many countries. Many slender constructions use hot-dip galvanized reinforcement to avoid spalling which leads to expensive repair. It is also worth noting that concrete debris that may fall fom a cracked surface may cause serious harm, especially in urban areas.
Designers have been known to request that the section of a steel construction that is to be set into concrete should be free from zinc. This request is totally unnecessary and to prevent one part of a construction from zinc coating during galvanizing is often more expensive than galvanizing the whole construction. Designers should reassured that the adherence between the hot-dip galvanized surface and the concrete is normally so strong that a sledge hammer is needed to separate them.
Zinc has been used as sacrificial anode to protect ships’ hulls, harbour constructions, cisterns and similar structures against corrosion. Of the available metallic coatings hot-dip galvanizing has been shown to be the most durable and technically suitable. Hot-dip galvanizing of reinforcement steel for concrete has been used worldwide for many years. Even in very severe conditions this surface treatment has shown to be a reliable choice.
Detailed studies, for example in Australia and at RISE KIMAB (Corrosion- and Metals Research Institute) in Sweden have shown the following results:
- Accelerated corrosion only takes place during the first hours after pouring the concrete. After that, the coating is passivated. The loss of zinc is low, in the range 2-5 µm.
- Zinc gives cathodic protection on exposed steel surfaces, which is a benefit when cutting or welding the reinforcement or when it is mechanically damaged.
- The adherence between reinforcement steel and the concrete is good.
- Concrete spalling does not occur.
- The risk for discolouration of the facade due to rust runs is eliminated.
- By galvanizing it is possible to use reinforced concrete in more aggressive environments.
- Variations in concrete quality are reduced.
- Thinner concrete cover can be allowed.
When cost and other consequences of corrosion damage of a construction are analyzed, the extra cost for galvanizing is negligible. It could almost be considered as an insurance premium – which only has to be paid once.
Performance of Galvanized Rebar in Concrete
Newly mixed concrete consists primarily of aggregates, cement powder, and water, the latter two components forming cement paste, which soon harden by a process called ‘hydration.’ As the cement paste hardens it binds the aggregate as a solid matrix which gives concrete its load carrying ability and durability. The consumption of the mix water in the hydration-hardening reaction leaves capillary, and ‘gel’ pores in the concrete matrix through which atmospheric gasses, pollutants, and water can penetrate when the concrete is ‘wetted’ by rain, condensation, or spray. The retained water within the pores of the concrete matrix becomes saturated with the chemical components of the cement and forms a highly alkaline solution, with a nominal pH of ~12.5, depending on the specific cement powder used.
Bare reinforcing steel is normally passivated in the initial pH of the contained-water in newly hardened concrete, however, wetting and drying cycles allow for atmospheric gasses including carbon dioxide and sulfur dioxide to dissolve in the pore water, and their acidity in solution begins to lower the pH of the pore water. This process is called carbonation.
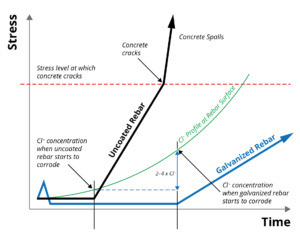
Bare reinforcing steel begins to loose its ‘passivation’ or dormancy as the pH surrounding the steel passes below about pH 11.5, and rusting of the steel begins and progresses, the resulting corrosion products take up more space than the steel consumed and this volume expansion within the constraining, rigid concrete matrix, is that substantial stresses are exerted on the surrounding concrete.
In addition to the lowering of the pH of pore space water below the threshold of passivation of imbedded bare steel, chloride ions from the structure surroundings, also are dissolved in the pore water, and once permeation down to the steel surface, further act to destroy the passivation of the embedded bare steel. The time elapsed before the combination of acidic atmospheric components and chlorides permeate to the embedded steel surface is a function of the environment, wetting cycles, porosity and composition of the concrete, and the length and difficulty of traversing the labyrinthine path to the bar surface. This latter factor is partly related to the ‘depth of cover’ of the embedded bar.
How Zinc Protects Rebar in Concrete
The corrosion protection afforded by galvanized rebar in concrete is due to a combination of beneficial effects. Of primary importance is the substantially higher chloride threshold (2-4 times) for zinc coatings to start corroding compared to uncoated steel. In addition, zinc has a much greater pH passivation range than steel, making galvanized rebar resistant to the pH lowering effects of carbonation as the concrete ages. Even when the zinc coating does start to corrode, its corrosion rate is considerably less than that of uncoated steel.
Why Galvanized Rebar Maintains Concrete’s Integrity
Zinc’s corrosion products are loose, powdery minerals that are less voluminous than iron corrosion products and are able to migrate away from the galvanized rebar surface into the adjacent concrete matrix. As a result, corrosion of the zinc coating causes very little physical disruption to the surrounding concrete. The elemental map (left) is evidence of this migration. The white spots in the concrete indicate zinc oxide which has migrated away from the galvanized rebar/concrete interface.
There is also evidence to suggest that the diffusion of zinc’s corrosion products helps fill pore spaces at the concrete/rebar interface, making this area less permeable and helps to reduce the transport of aggressive species such as chlorides through this interface zone to the zinc coating. The reactions between zinc and concrete, and the resulting corrosion product diffusion, also explains why galvanized rebar has such good bond strength with concrete.
Tolerance of Lower pH due to Carbonation
Zinc remains passive at significantly lower pH levels than for black steel (9.5 versus 11.5) making galvanized rebar far less susceptible to corrosion due to carbonation of the concrete.
Hot-dip galvanized reinforcement in chloride environments
Practical experiments performed by RISE KIMAB have shown that zinc copes very well even in chloride-containing environments. Up to 1.5 wt. % chloride in the concrete leads to negligible corrosion of the galvanized rebar. Conversely, unprotected reinforced steel had difficulty to cope with this chloride concentration and was corroded. Zinc can withstand even higher chloride concentrations, but with a related decrease in coating life. In such an environment unprotected steel in addition to normal corrosion also will show pitting corrosion, which does not occur on galvanized steel. Also in carbonated concrete, galvanized steel is more durable than unprotected steel.
Hot dip galvanized reinforcement is a reliable partner in concrete technology. It minimizes the risk of steel corrosion and concrete spalling and gives a strong and cost effective contribution to the durability of the concrete.
Zinc’s Initial Reaction in Fresh Concrete
Zinc reacts with wet concrete to form calcium hydroxyzincate accompanied by the evolution of hydrogen. This corrosion product is insoluble and protective of the underlying zinc (provided that the surrounding concrete mixture is below a pH of about 13.3). Research has shown that during this initial reaction period until coating passivation and concrete hardening occurs, some of the pure zinc layer of the coating is dissolved. However, this initial reaction ceases once the concrete hardens and the hydroxyzincate coating has formed. Studies of galvanized rebar recovered from field structures indicate that the coating remains in this passive state for extended periods of time, even when exposed to high chloride levels in the surrounding concrete.
For concretes of high pH, or where some background chlorides are expected, the zinc surface can be passivated, using a range of proprietary post treatments, as a safeguard against excessive hydrogen evolution that may, in serious cases, reduce the pullout strength of the bar. For normal concrete conditions, research has shown no statistical difference in bond strength between galvanized rebar that was passivated and not passivated.
Corrosion Model
Zinc coatings have a higher chloride (Cl -) corrosion threshold (2-4 times) than that of uncoated steel, significantly extending the time until corrosion initiation. Once corrosion of the zinc coating does occur, the properties of the corrosion products and their ability to migrate into the concrete matrix reduces stress generation in the surrounding concrete, further extending the life of the reinforced concrete structure. Black and galvanized rebar corrosion performance in concrete can be shown graphically at right.
Bending Galvanized Rebar prior to Galvanizing
Hot-dip galvanized reinforcing steel can either be bent prior to galvanizing or after the galvanized coating has been applied. When bending prior to galvanizing, it is recommended that the bend radii be as large as possible in order to avoid accelerated aging of the steel by cold working.
Bond Strength
Arguably the most important property contributing to a successful reinforced concrete structure is the bond strength between the reinforcing steel and the concrete. A strong bond must be developed in order for the concrete to achieve its designed capacity. Factors that influence the strength or reinforced concrete include, but not limited to, are bar diameter, the absence or presence of bar surface deformations (ribs), the geometry of the ribs, concrete cover over the bars, and the orientation in the concrete matrix.
Good bonding between reinforcing steel and concrete is essential for reliable performance of reinforced concrete structures. When protective coatings on steel are used, it is essential to ensure that these coatings do not reduce bond strength. Studies on the bonding of galvanized and black steel bars to Portland Cement concrete have been investigated. The results of these studies indicate:
- Development of the bond between steel and concrete depends on age and environment
- In some cases, the time required for developing full bond strength between steel and concrete
may be greater for galvanized bars than for black, depending on the zincate/cement reaction - The fully developed bond strength of galvanized and black deformed bars is the same.
Mechanical Properties
Ductility and Yield/Tensile Strength
Ductility and strength of reinforcing steel are important to prevent brittle failure of reinforced concrete. Studies on the effect of galvanizing on the mechanical properties of steel reinforcing bars have demonstrated that the tensile, yield, and ultimate strength, ultimate elongation, and bend requirements of steel reinforcement are substantially unaffected by hot-dip galvanizing, provided proper attention is given to steel selection, fabrication practices and galvanizing procedures. The effect of the galvanizing process on the ductility of steel bar anchors and inserts after being subjected to different fabrication procedures has also been investigated. The results demonstrate conclusively that, with correct choice of steel and galvanizing procedures, there is no reduction in the steel’s ductility.
Fatigue Strength
An extensive experimental program examining the fatigue resistance of galvanized steel reinforcement shows that deformed reinforcing steel, exposed to an aggressive environment prior to testing under cyclic tension loading, performs better when galvanized.
Dissimilar Metal Contact
Another consideration when using galvanized reinforcement in concrete is the possibility of establishing a bimetallic couple between zinc and bare steel (i.e., at a break in the zinc coating or direct contact between galvanized steel and black steel bars) or other dissimilar metals. A bimetallic couple of this type in concrete should not be expected to exhibit corrosive reactions as long as the two metals remain passivated. To ensure this is the case, the concrete depth to the zinc/steel contact should not be less than the cover required to protect black steel alone under the same conditions.
Therefore, when galvanized reinforcement is used in concrete, it should not be coupled directly to large areas of black steel reinforcement, copper, or other dissimilar metal. Bar supports and accessories should be galvanized. Tie wire should be annealed wire, 16-gauge or heavier, preferably galvanized. If desired, polyethylene and other similar tapes can be used to provide insulation between dissimilar metals.
Reference: https://www.galvanizedrebar.com – International Zinc Association